Sodium Emission Spectrum

by Carlos Clarivan
Title
Sodium Emission Spectrum
Artist
Carlos Clarivan
Medium
Photograph - Photograph
Description
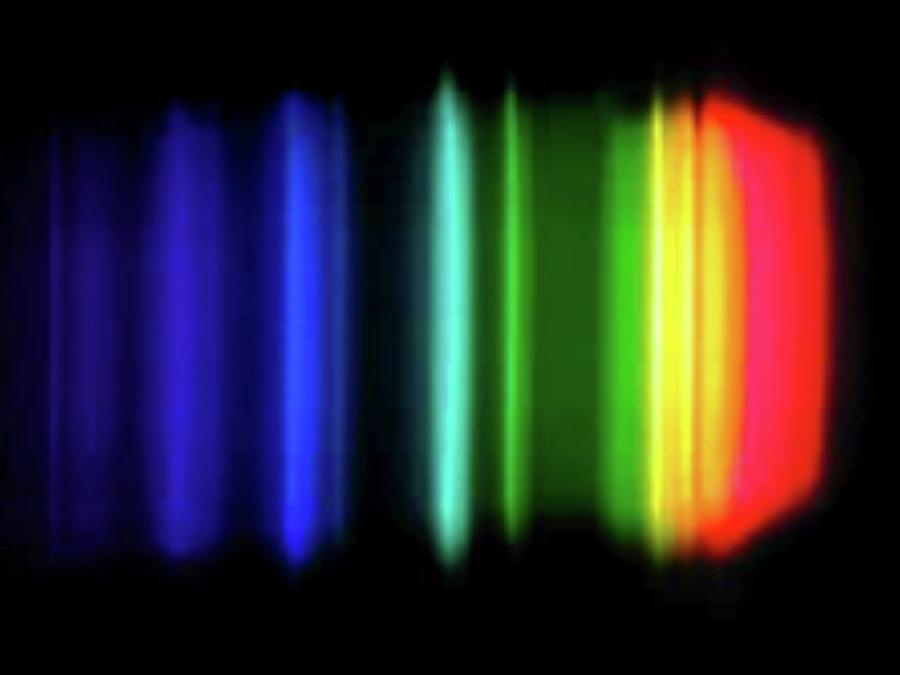
Sodium emission spectrum when reacting with water. The colours here show the electromagnetic radiation emitted during this reaction by the element sodium. Such spectra are used to identify chemical elements, both in the laboratory and in distant stars. This spectrum is dominated by the bright doublet known as sodium D-lines at 589.0 and 589.6 nanometres (nm). These lines are emitted in a transition from the 3p to the 3s atomic energy levels. The line at 589.0 has twice the intensity of the line at 589.6 nm. The strongest visible line other than the D-lines is the line at 568.8 nm. For this spectrum with a nanometre axis, see image C028/6300.
Uploaded
October 7th, 2018
Statistics
Viewed 1,247 Times - Last Visitor from New York, NY on 04/25/2024 at 8:47 PM
Embed
Share
Sales Sheet









































