Gold #2

by Carlos Clarivan/science Photo Library
Title
Gold #2
Artist
Carlos Clarivan/science Photo Library
Medium
Photograph - Photograph
Description
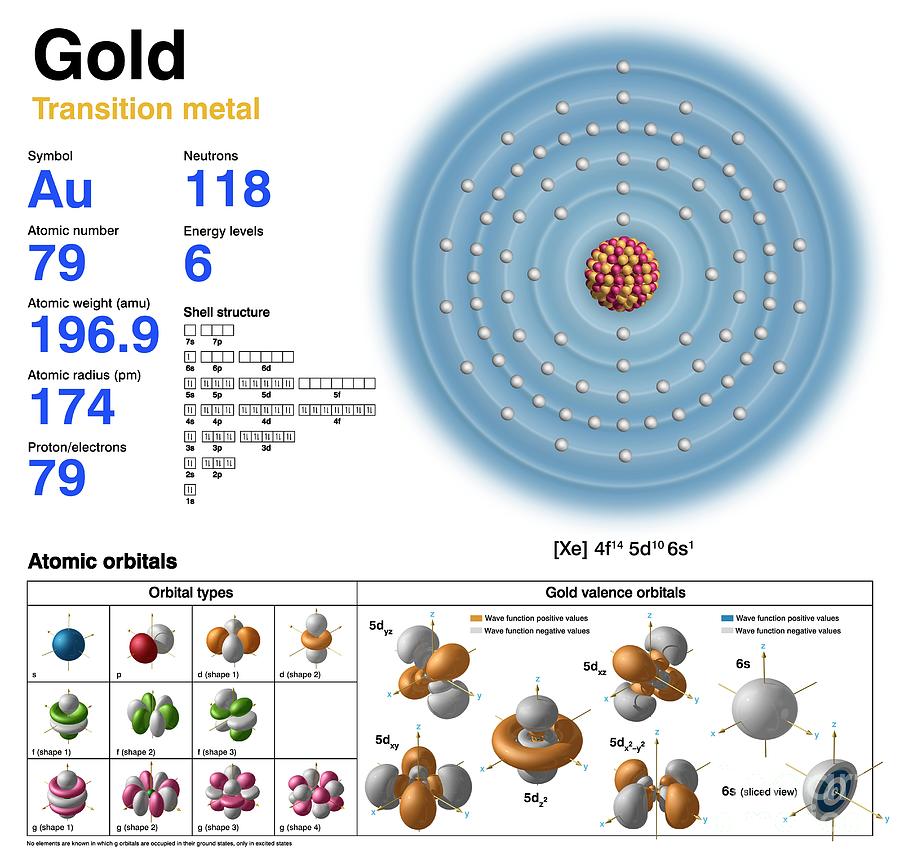
Gold (Au). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of gold-197 (atomic number: 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). Gold is a transition metal in group 11, period 6, and the d-block of the periodic table. It has a melting point of 1064 degrees Celsius. The trends across the transition metals are due to electrons filling an inner d-subshell (here, within the 5th ring), shielding the outer (valence) electrons from the increasing nuclear charge.
Uploaded
October 18th, 2019
Embed
Share









































