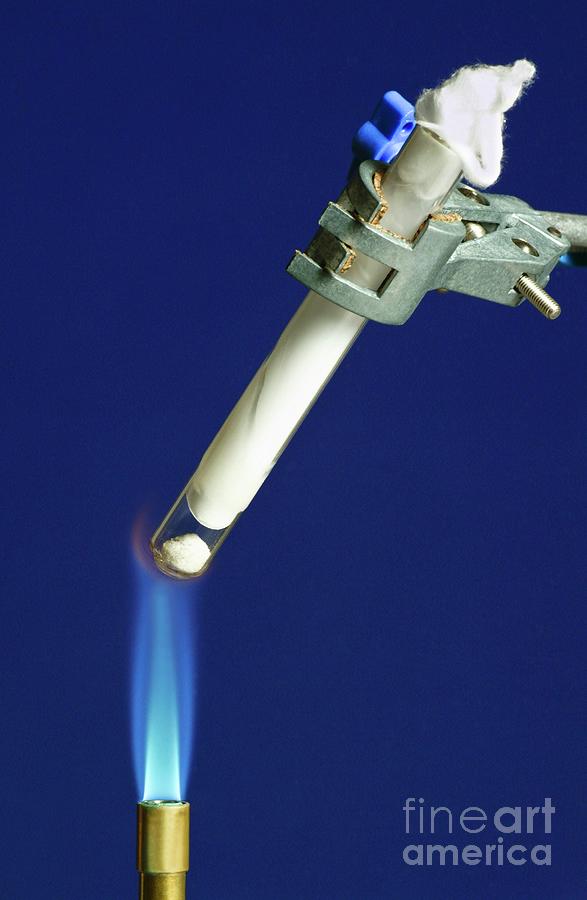
Ammonium Chloride Decomposition

by Martyn F. Chillmaid/science Photo Library
Title
Ammonium Chloride Decomposition
Artist
Martyn F. Chillmaid/science Photo Library
Medium
Photograph - Photograph
Description
Ammonium chloride decomposition. Test tube of ammonium chloride (NH4Cl) being heated over a bunsen burner flame. Ammonium chloride decomposes readily when heated, but condenses in the cooler area at the top of the test tube. This is a reversible reaction, where the ammonium chloride decomposes into the gases ammonia (NH3) and hydrogen chloride (HCl). These gases react together in the cooler regions of the test tube to reform the solid ammonium chloride.
Uploaded
May 9th, 2022
Statistics
Viewed 355 Times - Last Visitor from New York, NY on 04/16/2024 at 4:21 AM
Embed
Share
Sales Sheet
Comments
There are no comments for Ammonium Chloride Decomposition. Click here to post the first comment.






































